Junior Research Group Tumorigenesis and molecular cancer prevention
Dr. Karol Nowicki-Osuch
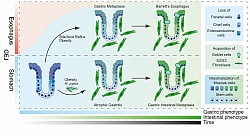
Schematic summary of unified developmental trajectories of Gastric and Oesophageal Adenocarcinoma. Both tumour types originate from stomach cells which acquire intestinal-like phenotype and become Barrett’s Oesophagus or Gastric Intestinal Metaplasia. Some of these lesions develop into cancer.
© dkfz.de
Gastric and oesophageal tumours are, jointly, the second most common cause of cancer death globally. The poor survival (approx. 15% 5-year survival) of patients diagnosed with these tumour types stems from the frequent late diagnosis. However, the early diagnosis of these tumours dramatically increases patients’ 5-year survival to over 75%. Our ability to detect early stages of gastric and oesophageal cancers is limited by our understanding of how these cancers develop. For decades, we have known that these cancers develop via precancerous lesions called Barrett’s Oesophagus and Gastric Intestinal Metaplasia. However, despite decades of clinical knowledge our molecular understanding of precancerous lesion and their relationship with cancer and, importantly, normal tissues they originate from is limited. The Tumorigenesis and Molecular Cancer Prevention Research Group uses state-of-art approaches to characterize and model early transcriptional, genetic and epigenetic processes that drive the development of gastro-oesophageal tumours and their precancerous lesions. A critical part of our work is to understanding how normal cells of the stomach and oesophagus lose their molecular identity and slowly transform into (pre)cancerous states. Using single-cell RNA sequencing, we demonstrated that, despite its name, Oesophageal Adenocarcinoma, a subtype of Oesophageal Cancer, originates from the stomach. It is also molecularly indistinguishable from other gastric cancers. Our work has also shown that the precancerous lesions have unique molecular properties that can only be described as single-cell molecular mosaicism – a state in which cells can stably maintain a mixed phenotype that is normally observed in two different cell types of the human body.
Our previous work demonstrated that the state-of-art single-cell sequencing approaches could accurately predict cell-of-origin as well as transcriptional and epigenetic make of (pre)cancerous lesions. In the next stage of our research, the Tumorigenesis and Molecular Cancer Prevention Research Group aims to use our existing expertise, approaches, and newly obtained knowledge about the molecular features of developing cancers to answer two questions:
- Can we model the earliest stages of gastro-oesophageal tumour development using in vivo and in vitro models?
- Can we use our detailed understanding of the changes that occur along the developmental trajectory of gastro-oesophageal cancers to improve patient’ survival through early cancer detection?
- Developing new organoid models of healthy and disease states and testing how genetic and epigenetic factors alter cellular behaviour;
- Using novel sequencing approaches (multimodal single cell assays and the newly developed mutREAD) to define minute differences between healthy and disease states;
- Developing novel computational approaches to data processing, analysis and visualisation;
- Implement of our technologies, early detection approaches and knowledge into clinically applicable settings in collaboration with clinical partners in Germany, Poland, the UK and the US.

