Research
- Research Topics
- Cell Biology and Tumor Biology
- Stem Cells and Cancer
- Inflammatory Stress in Stem Cells
- Experimental Hematology
- Molecular Embryology
- Signal Transduction and Growth Control
- Epigenetics
- Redox Regulation
- Vascular Oncology and Metastasis
- Clinical Neurobiology
- Molecular Neurogenetics
- Chaperones and Proteases
- Vascular Signaling and Cancer
- Molecular Neurobiology
- Mechanisms Regulating Gene Expression
- Molecular Biology of Centrosomes and Cilia
- Dermato-Oncology
- Pediatric Leukemia
- Tumour Metabolism and Microenvironment
- Personalized Medical Oncology
- Molecular Hematology - Oncology
- Cancer Progression and Metastasis
- Translational Surgical Oncology
- Neuronal Signaling and Morphogenesis
- Cell Signaling and Metabolism
- Cell Fate Engineering and Disease Modeling
- Cancer Drug Development
- Cell Morphogenesis and Signal Transduction
- Functional and Structural Genomics
- Molecular Genome Analysis
- Molecular Genetics
- Pediatric Neurooncology
- Cancer Genome Research
- Chromatin Networks
- Functional Genome Analysis
- Theoretical Systems Biology
- Neuroblastoma Genomics
- Signaling and Functional Genomics
- Signal Transduction in Cancer and Metabolism
- RNA Biology and Cancer
- Systems Biology of Signal Transduction
- Molecular thoracic Oncology
- Proteomics of Stem Cells and Cancer
- Computational Genomics and System Genetics
- Applied Functional Genomics
- Applied Bioinformatics
- Translational Medical Oncology
- Metabolic crosstalk in cancer
- Pediatric Glioma Research
- Cancer Epigenomics
- Translational Pediatric Sarcoma Research
- Artificial Intelligence in Oncology
- Neuropathology
- Pediatric Oncology
- Neurooncology
- Somatic Evolution and Early Detection
- Translational Control and Metabolism
- Soft-Tissue Sarcoma
- Precision Sarcoma Research
- Brain Mosaicism and Tumorigenesis
- Mechanisms of Genome Control
- Translational Gastrointestinal Oncology and Preclinical Models
- Translational Lymphoma Research
- Mechanisms of Leukemogenesis
- Genome Instability in Tumors
- Developmental Origins of Pediatric Cancer
- Brain Tumor Translational Targets
- Translational Functional Cancer Genomics
- Regulatory Genomics and Cancer Evolution
- SPRINT
- Cancer Risk Factors and Prevention
- Cancer Epidemiology
- Biostatistics
- Clinical Epidemiology and Aging Research
- Health Economics
- Physical Activity, Prevention and Cancer
- Preventive Oncology
- Digital Biomarkers for Oncology
- Genomic Epidemiology
- Cancer Survivorship
- Immunology and Cancer
- Cellular Immunology
- Molecular Oncology of Gastrointestinal Tumors
- T Cell Metabolism
- Translational Immunotherapy
- B Cell Immunology
- Immune Diversity
- Structural Biology of Infection and Immunity
- Applied Tumor-Immunity
- Neuroimmunology and Brain Tumor Immunology
- Adaptive Immunity and Lymphoma
- Immune Regulation in Cancer
- Systems Immunology and Single Cell Biology
- GMP & T Cell Therapy
- News
- Imaging and Radiooncology
- Radiology
- Research
- Computational Radiology Research Group
- Contrast Agents In Radiology Research Group
- Neuro-Oncologic Imaging Research Group
- Radiological Early Response Assessment Of Modern Cancer Therapies
- Imaging In Monoclonal Plasma Cell Disorders
- 7 Tesla MRI - Novel Imaging Biomarkers
- Functional Imaging
- Visualization And Forensic Imaging
- PET/MRI
- Dual- and Multienergy CT
- Radiomics Research Group
- Prostate Research Group
- Breast Imaging Research Group
- Bone marrow
- Musculoskeletal Imaging
- Microstructural Imaging Research Group
- Staff
- Patients
- Research
- Medical Physics in Radiology
- X-Ray Imaging and Computed Tomography
- Federated Information Systems
- Translational Molecular Imaging
- Medical Physics in Radiation Oncology
- Biomedical Physics in Radiation Oncology
- Intelligent Medical Systems
- Medical Image Computing
- Radiooncology - Radiobiology
- Radiation Oncology
- Molecular Radiooncology
- Nuclear Medicine
- Translational Radiation Oncology
- Molecular Biology of Systemic Radiotherapy
- Interactive Machine Learning
- Multiparametric methods for early detection of prostate cancer
- Molecular Mechanisms of Head and Neck Tumors
- Radiology
- Infection, Inflammation and Cancer
- Tumor Virology
- Viral Transformation Mechanisms
- Pathogenesis of Virus-Associated Tumors
- Immunotherapy and Immunoprevention
- Applied Tumor Biology
- Virotherapy
- Virus-associated Carcinogenesis
- Chronic Inflammation and Cancer
- Microbiome and Cancer
- Cell Plasticity and Epigenetic Remodeling
- Experimental Hepatology, Inflammation and Cancer
- Infections and Cancer Epidemiology
- Tumorvirus-specific Vaccination Strategies
- Mammalian Cell Cycle Control Mechanisms
- Molecular Therapy of Virus-Associated Cancers
- DNA Vectors
- Episomal-Persistent DNA in Cancer- and Chronic Diseases
- Other Units
- Cell Biology and Tumor Biology
- Research Groups A-Z
- Junior Research Groups
- Core Facilities
- Center for Preclinical Research
- Chemical Biology Core Facility
- Electron Microscopy
- Flow Cytometry
- Genomics and Proteomics
- Information Technology
- Library
- Kataloge -- Catalogues
- Zeitschriften - Journals
- E-Books - Ebooks
- Datenbanken - Databases
- Dokument-Lieferung - Document Delivery
- Publikationsdatenbank - Publication database
- DKFZ Archiv - DKFZ Archive
- Open Access
- Science 2.0
- Ansprechpartner - Contact
- More Information - Service
- Anschrift - Address
- Antiquariat - Second Hand
- Aufstellungssystematik - Shelf Classification
- Ausleihe - Circulation
- Benutzerhinweise - Library Use
- Beschaffungsvorschläge - Desiderata
- Fakten und Zahlen - Facts and Numbers
- Kooperationen, Konsortien - Cooperations, Consortia
- Kopieren, Scannen - Copying, Scans
- Kurse, Führungen - Courses, Introductions
- DKFZ-Intern - internal only
- DEAL-Info
- Light Microscopy
- Omics IT and Data Management Core Facility
- Small Animal Imaging
- Metabolomics Core Technology Platform
- Data Science @ DKFZ
- INFORM
- Baden-Württemberg Cancer Registry
- Cooperations & Networks
- National Cooperations
- International Cooperations
- Cooperational Research Program with Israel: DKFZ - MOST in Cancer Research
- Program
- Members of the Program Committee
- Call
- Publication Database
- German-Israeli Cancer Research Schools
- Archive
- Heidelberg - Israel, Science and Culture
- Symposium 40 Years of German-Israeli Cooperation
- 35th Anniversary Symposium
- 34th Meeting of the DKFZ-MOST Program
- 40th Anniversary Publication
- 30th Anniversary Publication
- 20th Anniversary Publication
- Flyer - The Cancer Cooperation Program
- List Publications 1976-2004
- Highlight-Projects
- Cooperational Research Program with Israel: DKFZ - MOST in Cancer Research
- Cooperations with industrial companies
- DKFZ PostDoc Network
- Cross Program Topic RNA@DKFZ
- Cross Program Topic Epigenetics@dkfz
- Cross Program Topic Single Cell Sequencing
- WHO Collaborating Centers
- DKFZ Site Dresden
- Health + Life Science Alliance Heidelberg Mannheim
Molecular and functional characterisation of the spindle position checkpoint in budding yeast
© dkfz.de
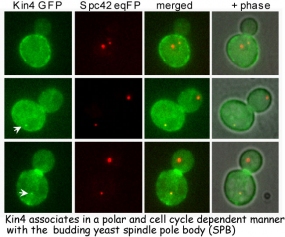
In all eukaryotic cells surveillance mechanisms, named checkpoints, make cell cycle progression dependent upon the successful completion of previous steps. Checkpoints are of extreme importance for the maintenance and accurate segregation of the genome. In the budding yeast, Saccharomyces cerevisiae, mitotic checkpoints ensure that each daughter cell will be provided with identical sets of chromosomes. The spindle position checkpoint (SPOC) senses any failure of cytoplasmic microtubules to correctly position the nucleus between mother and daughter cells. Thus making the transition out of mitosis and subsequent cell division (cytokinesis) dependent upon the correct partitioning of one set of chromosomes into the daughter cell. In the absence of SPOC, mutant cells defective in cytoplasmic microtubules undergo an aberrant mitosis and become aneuploid, reinforcing the importance of the SPOC in maintaining genome integrity (Figure 1).
The mitotic exit network (MEN) is a signalling transduction cascade that promotes exit from mitosis and cytokinesis. The SPOC inhibits the MEN via a two component GTPase activating protein (GAP) complex, composed of Bfa1 and Bub2. When the nucleus fails to move into the daughter cell, it is the function of Bub2-Bfa1 GAP complex to keep one of the most upstream components of the MEN, the Ras-like GTPase Tem1, in its GDP-inactive form until defects are corrected. Bfa1 and Bub2 associate with the yeast centrosome, called spindle pole body (SPB). Interestingly, Bfa1 and Bub2 are only found on the SPB that enters the daughter cell during mitosis. In response to cytoplasmic microtubules defects, Bfa1 and Bub2 are regulated in two ways. First, Bfa1 and Bub2 are re-located to both SPBs, a process that likely increases the GAP activity of the complex and makes Tem1 inactivation more efficient. Second, phosphorylation of Bfa1 by the polo kinase Cdc5, which decreases the GAP activity of the complex, is inhibited. Thus, localisation and GAP activity of Bub2-Bfa1 can be modulated by cytoplasmic microtubules. In this context it is interesting to note that Bfa1 and Bub2 directly bind to core SPB proteins involved in organisation of cytoplasmic microtubules. Recently, the Kin4 kinase has been identified as a novel component of the SPOC. In case of misalignment spindles, Kin4 phosphorylates Bfa1 and thereby inhibits its subsequent phosphorylation by Cdc5. In contrast to other mitotic regulators, Kin4 localises preferentially in the mother cell body. How Kin4 and Bub2-Bfa1 complex are able to respond to changes in cytoplasmic microtubule behaviour at a molecular level is unclear. Our work is therefore focusing on the regulation of Bub2-Bfa1, and on the identification and characterisation of novel components of the SPOC. For this, we are employing a combination of genomics, proteomics and advanced microscopy approaches.
