Cellular Therapies
CAR-T cell immunotherapy
Adoptive T cell therapy using genetically engineered T cells expressing a chimeric antigen receptor (CAR) has revolutionized the treatment modalities in leukemia and lymphoma for more than a decade now. However, the translation of the successes to solid tumor diseases is rather slow. Within our team, we are dedicated to the development and manufacturing of novel and safe CARs that can be applied in the clinics. For instance, we have developed two novel CAR-T cell products for the use in breast cancer patients that target the cancer testis antigen NY-BR-1 and prevent cross-reactivity against healthy brain tissue. Our team analyzed both for efficiency and safety in vitro and in vivo in a xenotransplant and target transgenic mouse model.
In close collaboration with Dr. Richard Harbottle (DKFZ, DNA Vector Lab), we have developed a novel DNA-plasmid-based vector system for the GMP-grade manufacturing of CAR-T cells that makes the use of (lenti)viral vectors dispensable. These vectors, which we named nS/MARt, do not possess the risk of genotoxicity but confer the same characteristics as viral vectors, e.g. stable expression (Bozza M et al, 2021, SciAdv, 7(16):eabf1333). As a desired side effect, this novel manufacturing method will reduce overall costs for CAR-T cell production. In another project, we are developing novel, specific CAR inhibitors that can temporarily impair their function and therefore maybe useful to lower clinical side effects. These inhibitors are based on AAV-particles that present matching CAR-epitopes on their surface.
CAR-library establishment
Image of a full opto-electric chip analyzed in the Berkeley Lights Lightning System. Jurkat-CAR-Venus-reporter cells were penned into cavities in single clones and have been activated specifically. Serial image analysis has been performed constantly from 4 – 24h post activation. Representative image after 16h of activation is shown.
© Patrick Schmidt
Another task that our team is working on, is the generation of a cell based CAR-library, which will allow the rapid screening for functional, high-quality CAR-T cells against novel or personalized targets. In this project, we combine rapid large-scale transfection technology using nS/MARt vectors with single cell based selection by the use of the Berkeley Lights Lightning System. We aim on developing a pre-selection strategy based on a highly sensitive reporter cell line and FACS sorting followed by multiplexed activation analyses on the opto-electric chip of the Lightning device.
AAViTOP Epitope Screening Platform and CARAAVs
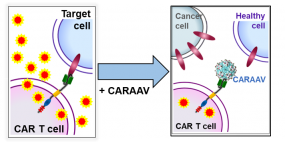
CARAAVs compete with cellular target for CAR-binding and thereby inhibit CAR-mediated T cell activity (i.e. cytokine secretion, killing of target cells).
© Silke Uhrig-Schmidt
While cancer immunotherapy with CAR-T cells highly effective, the modified T cells can be too active, or even react with target molecules on healthy tissue. This reaction can lead to life-threatening conditions requiring intensive care. Currently, the clinical management of severe side-effects only deals with the symptoms leaving the toxicity-causing CAR-T cells unaffected, or inducing their irrevocable destruction. Our innovative solutions are modified adeno-associated virus-based particles (CARAAVs) that offer a non-activating CAR-antagonism which is both fast and reversible. Making use of AAV capsid engineering, we generate virus-like particles (VLPs) that inhibit CAR-T cells by competing for CAR binding with the cellular target (see Figure). The epitope bound by a CAR-of-interest is cloned into the AAV cap sequence resulting in a small peptide insertion on the capsid surface. These AAVLPs are able to specifically bind to a CAR on CAR+ primary T cells and T cell lines, reduce cell surface presence of CARs and thereby decrease CAR-mediated killing activity of CAR-T cells in vitro and ex vivo. As certain amino acid constellations show a negative effect on capsid formation and are therefore underrepresented in commonly used random libraries, we further aim to define the limitations of AAV capsid assembly more clearly and develop strategies to overcome these limitations.
Since the epitope of a CAR is unknown in many cases, we developed a cell-free screening assay for epitope identification. The AAViTOP assay uses a library approach: First, overlapping fragments of the CAR target protein are cloned into the AAV capsid sequence followed by particle production. Second, the AAVLPs are tested for correct epitope presentation and binding using a soluble CAR/scFv or antibody and flow cytometry as a readout.
T cell receptor (TCR) discovery and T cell production
We aim for the development and routine application of personalized TCR gene-engineered adoptive T cell therapies (ACT) targeting individual- as well as shared patient-derived neoepitopes. Based on our implemented state-of-the-art tumor mutagenome analysis as well as in silico T cell epitope prediction pipeline that is combined with our highly sensitive peptide-loaded MHC-multimer-guided antigen-specific T cell discovery workflow (D121), we have already identified and validated several neoepitope-specific TCRs across various solid tumor identities. This TCR discovery workflow is further augmented by our own robust pMHC in-house production platform that outperforms commercially available reagents and allows us to provide complete coverage of the individual patients' HLA allotypes. By incorporating DNA-barcoded pMHC-I multimer reagents into the workflow, we link multiplexed tumor antigen recognition with their associated TCR repertoire and T cell differentiation phenotype at single cell resolution, which further improves the selection of TCR / antigen pairs for validation prior to their usage in an ACT.
Large-scale clinical grade T cell production
We have established manufacturing protocols for the GMP production of CAR-T cells and transgenic TCR-T cells using the semi-automated manufacturing platform CliniMACS Prodigy from Miltenyi Biotec. Protocols are available for the lentiviral vector based production as well as for production using DNA-plasmid vectors. With strong support by the Dietmar-Hopp-Foundation we envision to establish a TCR discovery lab and GMP manufacturing facility for cellular medical products in 2025. This will enable us to develop innovative therapy approaches and produce study drugs for Phase I trials at the NCT, the UKHD, for partner sites within the NCT network as well as to serve as manufacturer for approved cellular drugs.
T cell fitness
To deepen our knowledge about T cell (dys)function, we have built up a panel of analytical methods to determine T cell fitness of patients before and after T cell therapy by multiparametric flow cytometry. In an individual case, we could confirm the association between a high T cell fitness signature in the infusion product and long-term persistence of TCR-T cells in the patient with sustained reactivity to the tumor target ex vivo.
We further aim to monitor immune responses in the context of current standard of care therapies in clinical cohorts (e.g. PROMISE trial), with a focus on discerning naïve/memory/effector phenotypes as well as exhaustion biomarkers. Furthermore, we are analyzing the TCR repertoire in responders/non-responders in our clinical cohorts by deep sequencing of TCR variable beta (Vβ) (TCRB) chain. This will allow us to evaluate and measure the clonality and diversity of T cells that can arise in response to tumor-associated antigens and to investigate whether these changes could be predictive markers of response.
Resistance or escape mechanisms in cellular therapies
In addition to T cell fitness, we also explore other tumor-intrinsic resistance mechanisms with the goal to not only find resistance mechanisms to cellular therapies, but also to develop new tools to overcome them. For this, we test for resistance biomarkers, such as loss of (specific-haplotype) HLA-I expression and immunoediting of targeted (neo-)antigens, by cellular and molecular approaches. To test potential ways to overcome resistance we perform drug-screening and functional assays in in vitro models (cell lines) as well as in patient material, e.g. patient-derived tumor cell lines and explants.
Contact
CAR-T cell immunotherapy, CAR-library establishment & Large-scale clinical grade T cell production
Patrick Schmidt, PhD - patrick.schmidt@nct-heidelberg.de
AAViTOP Epitope Screening Platform and CARAAVs
Silke Uhrig-Schmidt, PhD - silke.uhrig-schmidt@nct-heidelberg.de
T cell receptor (TCR) discovery and T cell production
Marten Meyer, PhD - marten.meyer@dkfz-heidelberg.de
T cell fitness, Resistance or escape mechanisms
Maria Paula Roberti, PhD - mariapaula.roberti@nct-heidelberg.de
Publications
Bozza, M, De Roia A, Correia MP, Berger A, Tuch A, Schmidt A, Zörnig I, Jäger D, Schmidt P, Harbottle RP. A nonviral, nonintegrating DNA nanovector platform for the safe, rapid, and persistent manufacture of recombinant T cells. Science Advances, 7(16):eabf1333 (2021). doi: 10.1126/sciadv.abf1333.
Ran T, Eichmüller SB, Schmidt P, Schlander M. Cost of decentralized CAR T-cell production in an academic nonprofit setting. Int J Cancer. 147(12):3438-3445 (2020). doi: 10.1002/ijc.33156.
