Ultra-rare sarcoma
Limited availability of tumor samples, absence of model systems, lower number of patients for understanding of disease biology and drug development are critical hurdles in advancing basic, translational, and clinical research in rare cancers (<6 cases per 100,000/year) such as sarcoma. A subset of sarcoma, termed as ultra-rare sarcoma (≤1 case per 1,000,000, Stacchiotti S et al, 2021, Cancer, 27:2934) are exceedingly rare, with significant augmentation of above challenges. Our team aims to push forward ultra-rare sarcoma research by performing in-depth molecular characterization of tumor samples to inform diagnosis, classification, identification of pathognomonic alterations, model development, and entry points for biology-guided treatment.
Follicular Dendritic Cell Sarcoma

© DKFZ
One example of ultra-rare sarcoma is follicular dendritic sarcoma (FDCS) that originates from follicular dendritic cells (FDCs). Diagnosis of FDCS is difficult due to histological similarities with many epithelial, mesenchymal, meningeal, or lymphoid malignancies. FDCS patients have poor prognosis and are still commonly treated with a lymphoma chemotherapy regimen (CHOP) despite evidence of mesenchymal origin of FDCs. We have assembled a cohort of >30 FDCS samples and have characterized the genomic and transcriptomic landscape of alterations, which led to identification of previously not described targetable lesions and oncogenic mechanisms, such as BRAFV600E mutations and telomerase overexpression by enhancer hijacking. Results of our analysis have led to clinical benefit to FDCS patient and we have identified a rare histological subtype of this ultra-rare sarcoma. We continue to characterize this intriguing disease using DNA methylation landscape and spatial technologies.
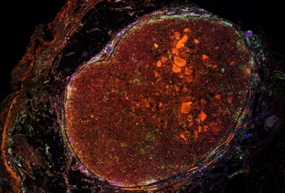
Alveolar soft-part sarcoma
© DKFZ
Alveolar-soft part sarcoma (ASPS) is yet another ultra-rare sarcoma with poor prognosis in the metastatic setting. ASPS is driven by (X;17) (p11;q25) translocation to form the ASPSCR1-TFE3 fusion oncoprotein, with low number of additional genomic alterations, such as mutations or structural variants. We were intrigued by the finding of high level of immune cell infiltration in ASPS tumors, which according to the current dogma, is not a frequent feature of tumors of "silent" genomes. Furthermore, certain patients with ASPS tumors show exceptional response to checkpoint inhibition, which, however, does not correlate with expression levels of immune checkpoint molecules. We are employing transcriptome-based immune profiling approaches and spatial proteomics to 1) uncover the secrets of immune cell chemotaxis, 2) identification of a biomarker for response to checkpoint blockade treatment and 3) identify new mechanisms and vulnerabilities of fusion-driven modulation of immune microenvironment. To this end, in collaboration with group of Ana Banito (Soft-tissue Sarcoma, DKFZ and KiTZ) we have developed an immunocompetent ASPS mouse model that will allow pre-clinical validation of targets and biomarkers.
